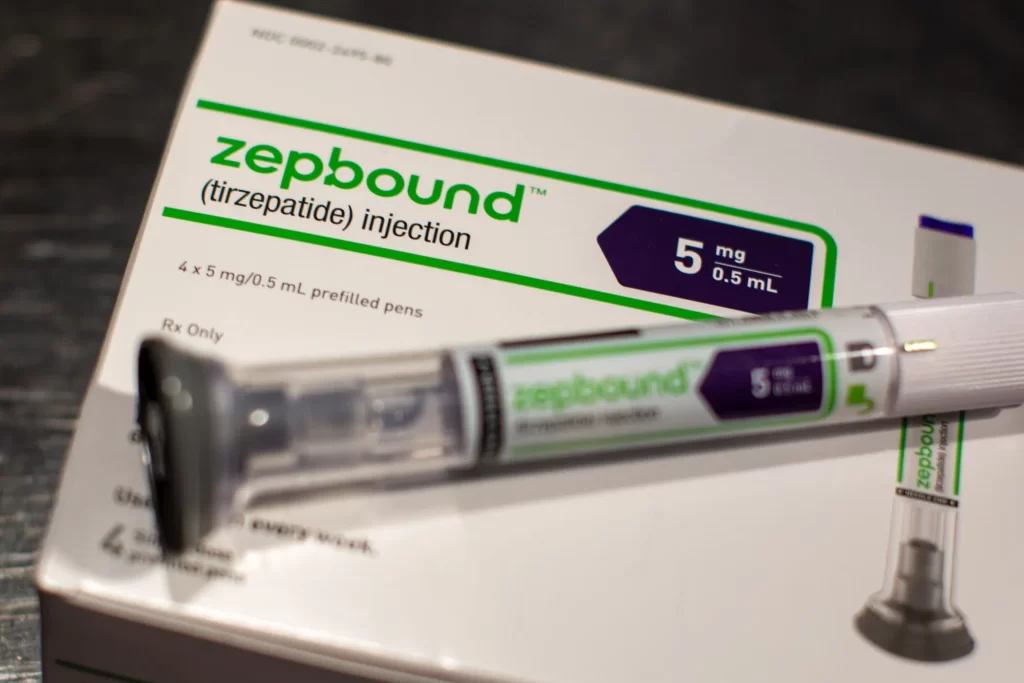
Eli Lilly (LLY) has achieved another milestone with its weight-loss drug, zepbund , which has now received FDA approval as a treatment for obstructive sleep apnea (OSA) in adults with obesity. This groundbreaking decision, announced on Friday, is expected to bring new hope to millions struggling with both excess weight and sleep-related breathing disorders.
The approval follows a series of clinical trials that demonstrated Zepbound’s remarkable ability to reduce breathing disruptions caused by OSA. Patients taking the medication saw a fivefold improvement over those who received a placebo, with nearly half experiencing a complete resolution of their sleep apnea symptoms. This marks a significant step forward in the treatment of OSA and its associated health risks.
Clinical Trials Prove Zepbound’s Effectiveness
Eli Lilly’s final phase trial focused on the drug’s ability to address both sleep apnea and weight loss. The study included patients using traditional positive airway pressure (PAP) masks as well as those who relied solely on Zepbound. Results showed that among patients who did not use a PAP mask, zepbund was about five times more effective than a placebo in reducing the number of breathing disruptions per hour.
The drug also contributed to significant weight loss, which plays a crucial role in mitigating sleep apnea. Patients who took zepbund alone lost an average of 45 pounds (18% of their body weight), while those who combined it with PAP therapy experienced an average weight loss of 50 pounds (20% of body weight).
According to Patrik Jonsson, president of Lilly USA, zepbund is the first-ever medication that provides substantial improvements for moderate-to-severe OSA while also promoting sustainable weight loss in adults struggling with obesity.
“Nearly half of the clinical trial participants saw such drastic improvements that they no longer showed symptoms of obstructive sleep apnea,” Jonsson said. “This represents a major breakthrough in managing the disease and reducing its long-term health risks.”
A Game Changer for the Weight-Loss and Sleep Apnea Market
Zepbund originally gained FDA approval for obesity treatment in November 2023. Since then, it has joined Eli Lilly’s other tirzepatide-based drug, Mounjaro, in dominating the rapidly growing weight-loss drug market.
Alongside Novo Nordisk’s popular medications Ozempic and Wegovy, these drugs have redefined the landscape for medical weight management, helping countless individuals shed excess pounds and improve their overall health.
The market reaction to Zepbound’s new FDA approval was swift. By midday Monday, shares of Eli Lilly had climbed 1.6%, continuing their impressive 34% year-to-date growth. On the other hand, ResMed (RMD)—a company specializing in sleep apnea devices like PAP masks—saw its stock drop by 4%, reflecting growing concerns that Zepbound’s success may cut into its traditional sleep apnea treatment market.
How Does zepbund Work for Sleep Apnea?
Zepbund is a tirzepatide-based medication, meaning it mimics hormones that regulate appetite and metabolism. By reducing body weight, it alleviates pressure on the airway, making it easier for individuals to breathe normally during sleep. The drug’s dual-action mechanism not only reduces weight but also improves sleep quality by minimizing breathing interruptions that characterize obstructive sleep apnea.
Doctors believe that for many patients, weight loss alone can significantly reduce OSA severity, and Zepbound is proving to be a powerful tool in achieving that goal.
FAQs
1. What is Zepbound used for?
Zepbund is an FDA-approved medication for weight loss and has now also been approved as a treatment for obstructive sleep apnea (OSA) in adults with obesity.
2. How does Zepbound help with sleep apnea?
By promoting substantial weight loss, zepbund reduces fat accumulation around the throat and airways, which is a leading cause of breathing disruptions in obstructive sleep apnea.
3. How much weight can I lose with Zepbound?
Clinical trials show that patients lose an average of 45 pounds (18% of body weight) on zepbund alone and 50 pounds (20%) when combined with PAP therapy.
4. Is Zepbound more effective than a CPAP machine?
Zepbund is not a replacement for CPAP therapy, but it has been shown to significantly reduce or even eliminate sleep apnea symptoms in many patients. Some trial participants no longer needed CPAP therapy after sustained weight loss.
5. What are the side effects of Zepbound?
Common side effects include nausea, vomiting, diarrhea, and decreased appetite. It’s important to consult a healthcare provider to determine if zepbund is right for you.
6. How long does it take for Zepbound to show results?
Weight loss and sleep apnea improvements can begin within weeks, but most patients see significant changes after several months of consistent use.
7. Is Zepbound covered by insurance?
Coverage varies by provider, but some insurance plans now cover zepbund for both obesity and sleep apnea treatments. Speak with your insurance company to check eligibility.
Conclusion
With FDA approval for sleep apnea treatment, Zepbound has the potential to revolutionize how doctors manage obesity-related sleep disorders. As a highly effective weight-loss drug that also reduces sleep apnea symptoms, it represents a major advancement in medical treatments for millions struggling with both conditions.
As zepbund continues to make waves in the weight-loss and sleep apnea market, it will be interesting to see how the landscape of OSA treatment evolves—especially with traditional PAP machines facing competition from pharmaceutical alternatives.
Stay in touch to get more news and updates on Buzzfeed!